Draw The Structure Of Cyclohexane
What is Conformation of Cyclohexane?
Cyclohexane has a not-polar construction that makes information technology almost free from ring strain. The well-nigh important conformations that it tin can have included chain conformation and boat conformation. The chair conformation is more than stable than the boat conformation. The boat conformation tin sometimes be more than stable than information technology is ordinarily, by a slight rotation in the C-C bonds and is chosen the skew boat conformation. Nevertheless, the chair conformation is the most stable cyclohexane form.
A Conformation of cyclohexane tin refer to many 3-Dimensional shapes assumed by a cyclohexane molecule without disturbing the integrity of the chemic bonds in it.
Table of Contents
-
- Conformations of Cyclohexane
- Boat and Chair Forms of Cyclohexane
- Stability of conformations of cyclohexane
- Recommended Videos
- Frequently Asked Questions–FAQs
Conformations of cyclohexane
A regular hexagon shape contains internal angles of 120o. However, the carbon-carbon bonds belonging to the cyclohexane ring take a tetrahedral symmetry, with the bond angles respective to 109.5o.
This is the reason why the cyclohexane band has a tendency to take up several warped conformations (so that the bail angles are brought closer to the tetrahedral angle (109.5o) and there is reduced overall strain energy).
Examples of common conformations of cyclohexane include the boat, the twist-gunkhole, the chair, and the half-chair conformations, which are named based on the shape that the cyclohexane molecule assumes in them.
These four cyclohexane conformations have been illustrated below forth with some insight into their stability.
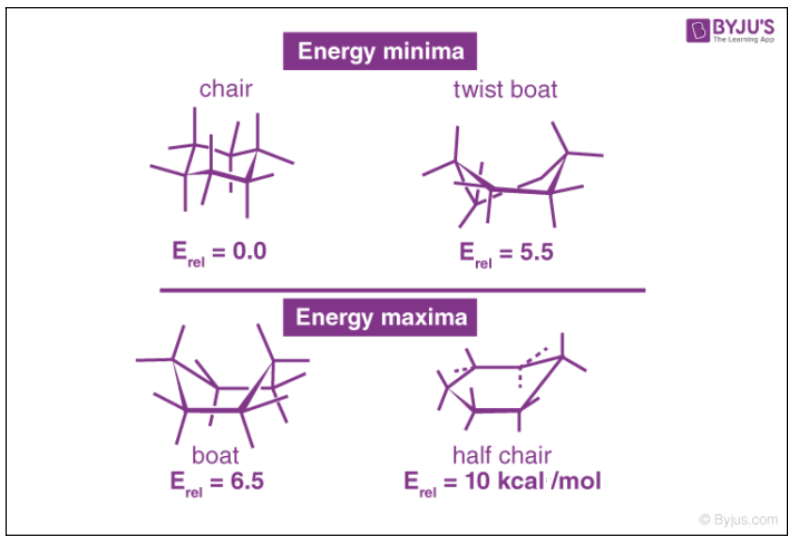
It can be noted that the cyclohexane molecule has the power to switch between the conformations listed to a higher place and that just the chair and the twist-boat conformations can be isolated into their respective pure forms.
Due to hydrogen-hydrogen interactions in these conformations, the bond length and the bond bending vary slightly from their nominal values.
The chair conformations of cyclohexane have lower energies than the boat forms. However, the rather unstable boat forms of cyclohexane undergo rapid deformation to requite twist-boat forms which are the local minima corresponding to the total energy.
The hydrogen atoms belonging to the carbon-hydrogen bonds that are at a perpendicular angle to the mean aeroplane are called axial hydrogens, whereas those belonging to the carbon-hydrogen bonds which are parallel to the mean airplane are called equatorial hydrogens. These bonds are besides referred to as axial and equatorial bonds respectively.
Boat and Chair Forms of Cyclohexane
Cyclohexane is the most widely occurring ring in compounds of natural origin. Its prevalence, undoubtedly a result of its stability, makes it the most important of the cycloalkanes. The departure of bond angle in cyclohexane molecules is more than in cyclopentane, information technology should be more than strained and less reactive than cyclopentane. Just actually, information technology is less strained and more stable than cyclopentane.
In social club to avoid the strain, cyclohexane does not exist as a planar molecule as expected. It exists equally a puckered band which is non-planar and the bond angles are shut to tetrahedral bond angles. Two such puckered rings for cyclohexane are called the boat and chair conformations.
Stability of conformations of cyclohexane
Generally, in the chair-shaped conformation of cyclohexane, at that place are two carbon-hydrogen bonds of each of the post-obit types:
-
-
-
- Axial 'up'
- Axial 'down'
- Equatorial 'upward'
- Equatorial 'down'
-
-
This geometry of chair cyclohexane conformations is generally preserved when the hydrogen atoms are replaced by halogen atoms such as fluorine, chlorine, bromine, and iodine. The phenomenon wherein the cyclohexane molecule undergoes a conversion from one chair form to a different chair form is called chair flipping (or ring flipping).
An illustration detailing chair flipping is provided below.

When chair flipping occurs, axial carbon-hydrogen bonds become equatorial and the equatorial carbon-hydrogen bonds become axial. However, they retain the respective 'upwards' or 'downward' positions.
It can be noted that at a temperature of 25o Celsius, 99.99% of the molecules belonging to a given cyclohexane solution would correspond to a chair-blazon conformation.
The gunkhole conformation of cyclohexane is not a very stable form due to the torsional strain applied to the cyclohexane molecule. The stability of this form is further affected past steric interactions between the hydrogen atoms. Owing to these factors, these conformations are mostly converted into twist-boat forms which have a lower torsional strain and steric strain in them.
These twist-boat conformations of cyclohexane are much more stable than their boat-shaped counterparts. This conformation has a concentration of less than 1% in a solution of cyclohexane at 25o. In order to increase the concentration of this conformation, the cyclohexane solution must be heated to 1073K and and then cooled to 40K.
Recommended Videos
Frequently Asked Questions – FAQs
Which conformation of cyclohexane is chiral?
Cyclohexane conformation gratis of bending strain: chair conformation is achiral because information technology has a centre of symmetry because boat conformation is achiral because information technology has a plane of symmetry. Twist boat conformation is chiral, since at that place is no element of symmetry.
Which is the most stable conformation of cyclohexane?
The chair grade shown to the correct is the about stable conformation of cyclohexane. The C-C-C bonds are very like to 109.5o, so they are well-nigh free from angle pressure. Information technology is also a completely staggered conformation and is, therefore, gratis of torsional stress.
Which conformation of cyclohexane is the least stable?
Gunkhole conformation is the least stable, with the highest strength, has steric hindrance on carbon 1 and carbon 4 between the two equatorial hydrogens, and has torsional stress, as each bail almost fully ellipses other bonds in the Newman projection.
Are diastereomers optically active?
Optical activity is the ability of a substance to rotate the plane polarised light. Chirality is the necessary condition for optical activity. Diastereomers lack mirror symmetry and are optically agile.
Which conformation is more stable, axial or equatorial?
Since axial bonds are parallel to each other, substituents larger than hydrogen typically suffer from greater steric crowding when centric rather than equatorial driven. Replaced cyclohexanes would therefore preferentially follow conformations in which the larger substituents assume an equatorial orientation.
Draw The Structure Of Cyclohexane,
Source: https://byjus.com/chemistry/conformation-of-cyclohexane/
Posted by: kelleyandon1984.blogspot.com


0 Response to "Draw The Structure Of Cyclohexane"
Post a Comment